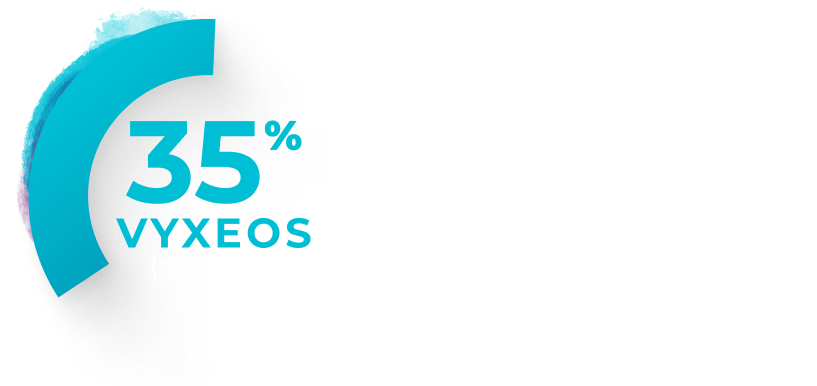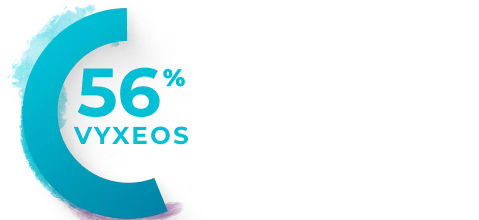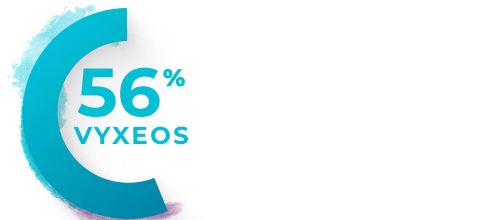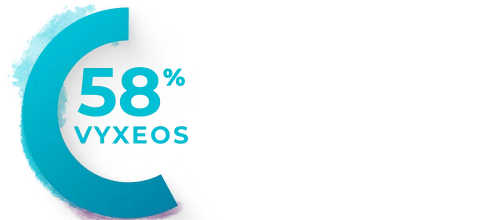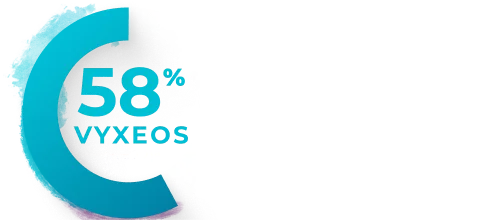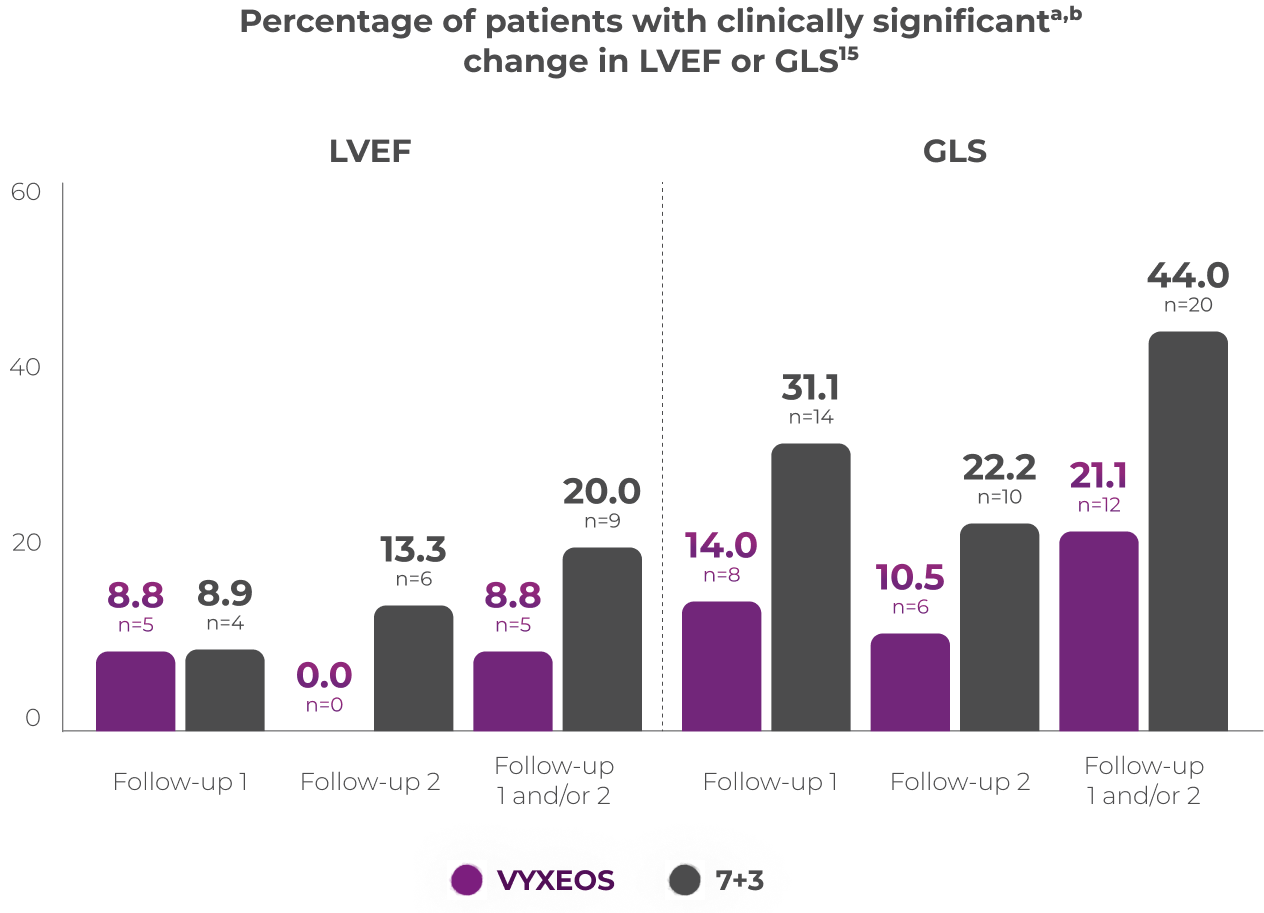
Percentage of patients with clinically
significanta,b change in LVEF or GLS15
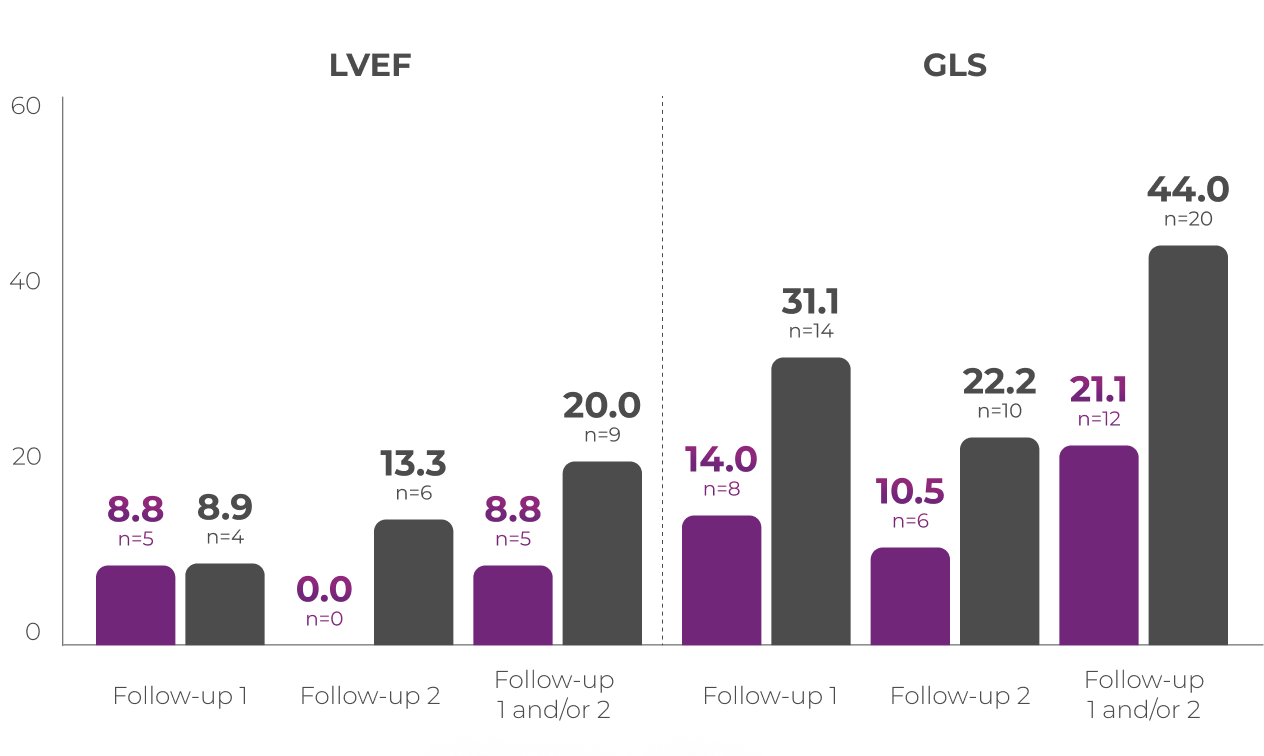

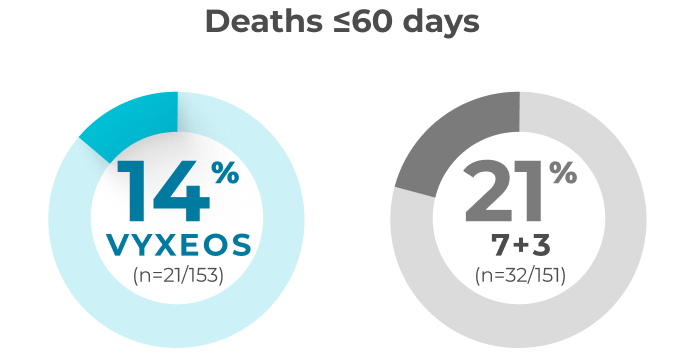
Patients from the Phase 3 study (VYXEOS [n=57]; 7+3 [n=45]) with normal baseline LVEF and at least one postbaseline echocardiogram measure were evaluated. Patients with prior cumulative anthracycline exposure of >368 mg/m2 daunorubicin (or equivalent) were excluded from the study.15
Cardiac function was assessed at15:
- Baseline (treatment initiation)
- Follow-up 1: 30-45 days from last induction or prior to consolidation or salvage therapy, and/or
- Follow-up 2: Day 150 or 45 days from last treatment (whichever was later)
Median baseline LVEFa and GLSb in the VYXEOS and 7+3 arms were15:
- LVEF: VYXEOS, 63.3; 7+3, 62.0
- GLS: VYXEOS, -21.3; 7+3, -21.1
Limitations of subanalysis
The limitations of this analysis include its post hoc design, the restricted patient population, and limited follow-up time points. No conclusions should be drawn. Analysis was not powered to determine statistical significance.15
VYXEOS treatment is not recommended in patients with cardiac function that is less than normal. Discontinue VYXEOS in patients with impaired cardiac function unless the benefit of continuing treatment outweighs
the risk.1