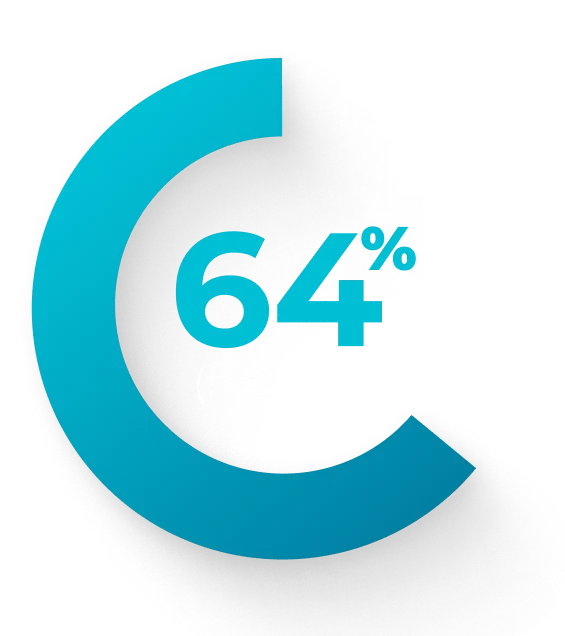
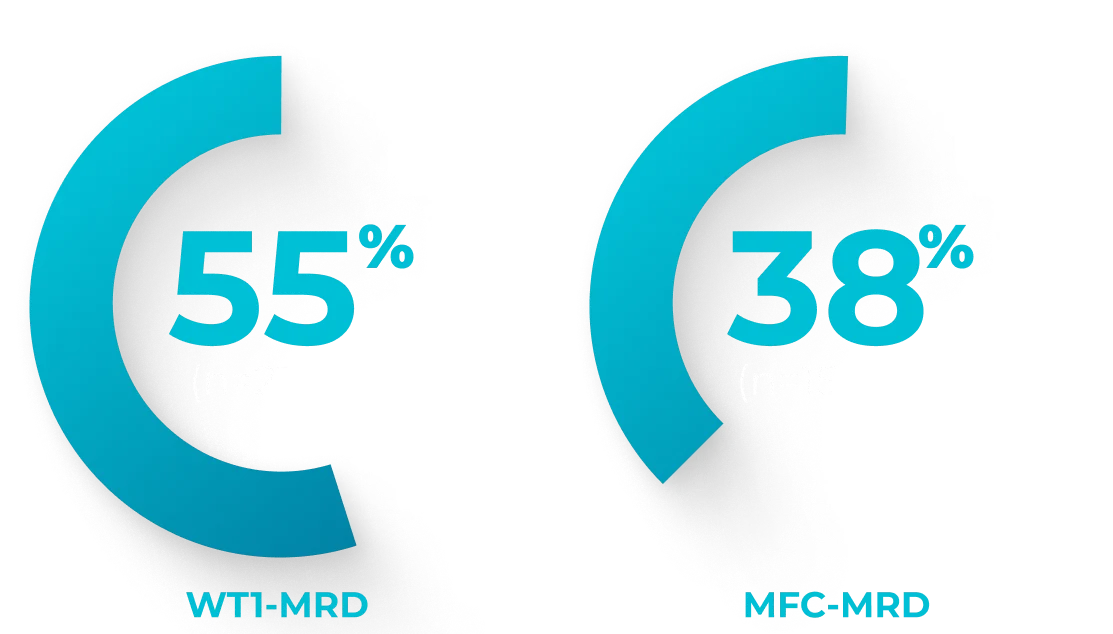
Retrospective chart review (N=188) of newly diagnosed patients median age 65 years (range: 26-80) with AML-MRC or t-AML treated with VYXEOS across 25 German centers between 2018 and 2020. Dosing was administered per the EU label.a Patient characteristics were similar to the Phase 3 study, but the treatment course substantially differed in that a much lower frequency (14%) received second induction with VYXEOS compared with 31% in the Phase 3 study. Of patients who achieved CR/CRi (n=85) after induction, 36 were tested for MRD at the time of transplant.
Safety (n=188) was consistent with the Phase 3 study. Day 30 mortality after VYXEOS induction (n=176) was 8% (60-day mortality was not reported). Grade 3/4 nonhematologic toxicities included infection (22%), pneumonia (22%), febrile neutropenia (15%), gastrointestinal (4%), bleeding (4%), and renal failure (3%). In patients with CR/CRi, recovery to ANC ≥500/μl and platelet count ≥50,000/μl was observed in 95% and 92% of patients, respectively. Median time to ANC and platelet recovery was 33 days (range: 6-99 days) and 30 days (range: 7-77 days), respectively.



 Back to clinical data
Back to clinical data